QSAR, Molecular Docking Study, Drug- Likeness and ADMET of Tanshinone Derivatives as Anti- prostate Cancer Compounds
DOI:
https://doi.org/10.26438/ijsrcs.v12i3.194Keywords:
QSAR, ADMET, LNCap, Prostate cancer, Docking, Drug- likenessAbstract
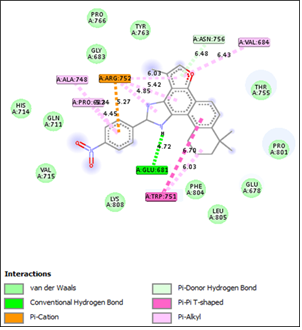
Cancer remains one of the leading causes of death worldwide, with the global cancer mortality rate continuing to rise. Projections estimate approximately 12 million deaths by 2030. Prostate cancer, in particular, stands out as a major contributor to cancer-related mortality, with its incidence steadily increasing over the past decade. This study aims to construct a quantitative structure- activity relationship (QSAR) model for the development of a highly effective and robust anti-prostate cancer agent targeting the LNCaP cell line. Model One was found to be predictive, powerful, and reliable, based on the following statistical parameters: coefficient of determination (R²) = 0.9916, adjusted R² = 0.9878, standard error of estimation (SEE) = 0.346, mean absolute error (MAE) = 0.035, and concordance correlation coefficient (CCC) = 0.9238. Additionally, the model demonstrated anticancer activity of tanshinone derivatives based on the THSA, MLFER_E, ASP-3, AVP-7, and TDB1v descriptors. Molecular docking results revealed that the docking scores of the three most promising compounds ranged from (-10.4 to -10.6) kcal/mol. These findings suggest that the selected compounds hold significant potential for predicting the anti-proliferative effects of other tanshinone derivatives against the LNCaP prostate cancer cell line.
References
Solomon, V. R.; Hua, C.; Lee, H., “Hybrid pharmacophore design and synthesis of isatin-benzothiazole analogs for their anti-breast cancer activity,” Bioorg. Med. Chem., Vol.17, pp 7585-7592. 2009.
Chandrappa S, Kavitha CV, Shahabuddin MS, Vinaya K, Ananda Kumar CS, Rangantha SR., “synthesis of 2-(5-(4- chlorophenyl)furan-2-yl)methylene)-4-oxo-2-thiooxo-thiazolidin-3-yl)acetic acid derivatives and evaluation of their cytotoxicity and induction of apoptosis in human leukemia cells,” Bioorganic & Medicinal Chemistry, Vol.17, Issue.6, pp.2576- 84. 2009
Al-Suwaidan IA, Abdel-Aziz AA-M, Shawer TZ, Ayyad RR, Alanazi AM, El-Morsy AM, Mohamed MA, Abdel-Aziz NI, El-Sayed MA-A, El-Azab AS., “Synthesis, antitumor activity and molecular docking study of some novel3-benzyl-4 (3H) quinazolinone analogues,” Journal of Enzyme Inhib Med Chem., Vol.31, Issue.1, pp.78–89. 2016.
Nelen, V., “Epidemiology of Prostate Cancer. In: Ramon, J., Denis, L.J. (eds) Prostate Cancer,” Recent Results in Cancer Research, Vol.175, Springer, Berlin, Heidelberg, pp.1- 8. 2007.
Rawla P., “Epidemiology of prostate cancer,” World J Oncol., Vol.10, pp.63-89. 2019.
Siegel, R., Naishadham, D. and Jemal, A., “Cancer Statistics,” CA: A Cancer Journal for Clinicians, Vol.63, pp.11 - 30. 2013.
Siegel, R.L., Miller, K.D. and Jemal, A., “Cancer Statistics,” 201. CA: A Cancer Journal for Clinicians, Vol.65, pp.5- 29. 2015.
G. Attard, C. Parker, R. A. Eeles, F. Schr¨oder, S. A Tomlins, I. Tannock, C. G. Drake and J. S. de Bono, “Prostate cancer,” Lancet. Vol.387, pp.70- 82, 2016.
Horoszewicz JS, Leong SS, Kawinski E, Karr JP, Rosenthal H, Chu TM, Mirand EA, Murphy GP., “LNCaP model of human prostatic carcinoma.,” Cancer Res., Vol.43, Issue.4, PMID: 6831420, pp.1809-18. 1983.
van Bokhoven A, Varella-Garcia M, Korch C, Johannes WU, Smith EE, Miller HL, Nordeen SK, Miller GJ, Lucia MS., “Molecular characterization of human prostate carcinoma cell lines,” Prostate, Vol.57, Issue.3,PMID: 14518029 pp.205-25.2003.
Leelananda, Sumudu P., and Steffen Lindert, “Computational methods in drug discovery,” Beilstein journal of organic chemistry, Vol.12, Issue.1, pp.2694-2718, 2016.
Hillisch A, Pineda LF, Hilgenfeld R., “Utility of homology models in the drug discovery process,” Drug Discov Today, Vol.9, Issue.15 PMID: 15279849 pp.659- 69. 2004.
Siegel RL, Miller KD, Jemal A., “Cancer statistics,” CA Cancer J Clin., Vol.66, Issue.1, PMID: 26742998 pp.7-30. 2016.
Wojciechowska U, Didkowska J., “Illness and deaths from ma-lignant tumors in Poland,” National Cancer Registry, Cancer Cen-tre - Institute for them. Maria Sklodowska-Curie, ISSN 0867–8251. 2013.
Li H, Li X, Bai M, Suo Y, Zhang G, Cao X., “Matrine inhibited proliferation and increased apoptosis in human breast cancer MCF-7 cells via up regulation of Bax and down regulation of Bcl-2,” Int J Clin Exp Pathol., Vol.8, Issue.11, PMID: 26823806 pp.14793-9. 2015.
Walczak JR, Carducci MA., “Prostate cancer: a practical app. roach to current management of recurrent disease,” Mayo Clin Proc, Vol.82, pp.243– 249. 2007.
Huang SY, Chang SF, Liao KF, Chiu SC., “Tanshinone IIA Inhibits Epithelial-Mesenchymal Transition in Bladder Cancer Cells via Modulation of STAT3-CCL2 Signaling,” Int J Mol Sci., Vol.18, Issue.8, pp.1616. PMID: 28757590, 2017.
Munagala R, Aqil F, Jeyabalan J, Gupta RC., “Tanshinone IIA inhibits viral oncogene expression leading to apoptosis and inhibition of cervical cancer,” Cancer Lett., Vol. 356(2 Pt B): PMID: 25304375, pp.536-46. 2015.
Chang TW, Lin CY, Tzeng YJ, Lur HS., “Synergistic combinations of tanshinone IIA and trans-resveratrol toward cisplatin-comparable cytotoxicity in HepG2 human hepatocellular carcinoma cells,” Anticancer Res., Vol. 34, Vol.10, PMID: 25275043 pp.5473-80. 2014.
Wang Y, Song D, Costanza F, Ji G, Fan Z, Cai J, Li Q., “Targeted delivery of tanshinone IIA-conjugated mPEG-PLGA-PLL-cRGD nanoparticles to hepatocellular carcinoma,” J Biomed Nanotechnol, Vol.10, Issue.11, PMID: 26000384, pp.3244-52. 2014.
Li, Chunlong, et al., “The interplay between autophagy and apoptosis induced by tanshinone IIA in prostate cancer cells,” Tumor Biology, Vol. 37, pp.7667-7674, 2016.
Ketola K, Viitala M, Kohonen P, Fey V, Culig Z, Kallioniemi O, Iljin K., “High-throughput cell-based compound screen identifies pinosylvin methyl ether and tanshinone IIA as inhibitors of castration-resistant prostate cancer,” J Mol Biochem., Vol.5, Issue.1, PMID: 27891324, pp.12-22. 2016.
Liu W, Zhou J, Geng G, Shi Q, Sauriol F, Wu JH., “Antiandrogenic, maspin induction, and antiprostate cancer activities of tanshinone IIA and its novel derivatives with modification in ring A., “J Med Chem.., Vol.55, Issue.2, PMID: 22175694, pp.971-975. 2012.
Xu D, Lin TH, Zhang C, Tsai YC, Li S, Zhang J, Yin M, Yeh S, Chang C. The selective inhibitory effect of a synthetic tanshinone derivative on prostate cancer cells,” Prostate., Vol.72, Issue.7, PMID: 21932429, pp.803-16, 2012.
Wang H, Su X, Fang J, Xin X, Zhao X, Gaur U, Wen Q, Xu J, Little PJ, Zheng W., “Tanshinone IIA Attenuates Insulin Like Growth Factor 1 -Induced Cell Proliferation in PC12 Cells through the PI3K/Akt and MEK/ERK Pathways,” Int J Mol Sci., Vol.19, Issue.9, pp.2719 PMID: 30213025. 2018.
Wang M, Zeng X, Li S, Sun Z, Yu J, Chen C, Shen X, Pan W, Luo H., “A Novel Tanshinone Analog Exerts Anti-Cancer Effects in Prostate Cancer by Inducing Cell Apoptosis, Arresting Cell Cycle at G2 Phase and Blocking Metastatic Ability,” Int J Mol Sci., Vol.20, Issue.18, pp.4459, PMID: 31510010. 2019.
Xu D, Hu H, Guan J, Da J, Xie Y, Liu Y, Kong R, Song G, Zhou H., “Synthesis of novel tanshinone derivatives for treatment of castration-resistant prostate cancer,” Chem Biol Drug Des., Vol.94, Issue.3, PMID: 31108007, pp.1656-1663. 2019.
Yunusa U, Umar U, Idris S, Kubo A, Abdullahi T., “Experimental and DFT computational Insights on the adsorption of selected pharmaceutical of emerging concern from water system Onto magnetically modified biochar,” Journal of the Turkish Chemical Society, Section A Chemistry, Vol.8, Issue.4, pp.1179- 1196. 2021
Yunusa, Umar, et al., “Theoretical Investigations and Experimental Studies of the Adsorption of Diethyl Phthalate and Di (2-ethylhexyl) Phthalate on Reduced Graphene Oxide,” Applied Journal of Environmental Engineering Science, Vol. 7, Issue.2, pp.151- 168. 2021.
Tropsha A, Bajorath Jr., “Computational methods for drug discovery and design, ACS Publications,” Journal of Medicinal Chemistry, Vol.59, Issue.1, 2015.
Beheshti A, Pourbasheer E, Nekoei M, Vahdani S., “QSAR modeling of antimalarial activity of urea derivatives using genetic algorithm- multiple linear regressions,” Journal of Saudi Chemical society, Vol.20, Issue.3, pp.282- 290. 2016.
Minovski N, Župerl Š, Drgan V, Novič M., “Assessment of app.licability domain for multivariate counter-propagation artificial neural network predictive models by minimum euclidean distance space analysis: a case study,” Anal Chim Acta., Vol.759. PMID: 23260674, pp.28-42. 2013.
Chirico N, Gramatica P., “Real external predictivity of QSAR models: how to evaluate it? Comparison of different validation criteria and proposal of using the concordance correlation coefficient,” J Chem Inf Model. Vol.51, Issue.9, PMID: 21800825, pp.2320-35. 2011.
Connolly, Michael L., “Analytical molecular surface calculation,” Applied Crystallography, Vol.16, Issue.5, pp.548-558. 1983.
Rojas, H., Ritter, C., & Pizzol, F. D., “Mechanisms of dysfunction of the blood-brain barrier in critically ill patients: emphasis on the role of matrix metalloproteinases,” Revista Brasileira De Terapia Intensiva, Vol.23, pp.222-227. 2011.
Wang, J., & Hou, T., “Recent advances on in silico ADME modelling,” Annual reports in computational chemistry, Vol.5, pp.101-127. 2009.
Ma XL, Chen C, Yang J., “Predictive model of blood-brain barrier penetration of organic compounds,” Acta Pharmacol Sin., Vol.26, Issue.4, PMID: 15780201, pp.500-12. 2005.
Yamashita S, Furubayashi T, Kataoka M, Sakane T, Sezaki H, Tokuda H., “Optimized conditions for prediction of intestinal drug permeability using Caco-2 cells,” Eur J Pharm Sci., Vol.10, Issue.3, PMID: 10767597, pp.195-204. 2000.
Horie, Kazutoshi, Fuxing Tang, and Ronald T. Borchardt, “Isolation and characterization of Caco-2 subclones expressing high levels of multidrug resistance protein efflux transporter,” Pharmaceutical research, Vol.20, pp.161-168, 2003.
Chen W, Tang F, Horie K, Borchardt RT, “CACO-2 cell monolayers as a model for studies of drug transport across human intestinal epithelium,” In: Lehr KM (ed) Cell culture models of biological barriers, Taylor and Francis, New York, pp 143–163, 2002.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Abdulrahman Ibrahim Kubo, Saminu M. A

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors contributing to this journal agree to publish their articles under the Creative Commons Attribution 4.0 International License, allowing third parties to share their work (copy, distribute, transmit) and to adapt it, under the condition that the authors are given credit and that in the event of reuse or distribution, the terms of this license are made clear.





