Method Development and Verification for Dissolution of Magnesium Oxide Tablets 400 mg by Titration
DOI:
https://doi.org/10.26438/ijsrcs.v12i1.181Keywords:
Dissolution, Magnesium Oxide Tablets, HPLC, Titration, Conductivity Suppression Detector (SCD), ICH guideline, Atomic Absorption Spectroscopy (AAS)Abstract
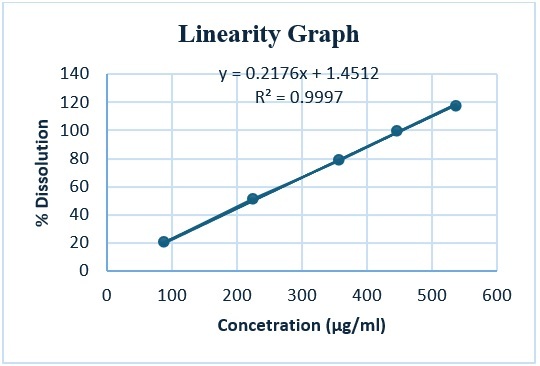
Dissolution analysis by HPLC for Magnesium Oxide Tablets 400 mg with Conductivity Suppression (SCD) Detector and/or by Atomic Absorption Spectroscopy as per current USP monograph method is quite difficult and non-feasible due to non-economical availability of SCD detector and Atomic Absorption Spectroscopy. Hence, Dissolution analysis by Titration method for Magnesium Oxide Tablets has been developed using 0.025M Edetate disodium VS as titrant for titration method. The Dissolution Tolerance is NLT (Q) 75% of the labelled amount of Magnesium Oxide is dissolved. The method was validated as per ICH Quality guidelines Q2(R2) Validation of Analytical Procedure with specificity, precision, linearity and accuracy. The study findings obtained the coefficient correlation of 0.9998 for linearity and %RSD of 2.9 with 91% of average dissolution for precision. The recovery results of 50%, 100% and 120% ensures the accuracy of the method with 100.1%, 98.1% and 97.0% respectively.
References
J.K. Aronson, Magnesium salts, Meyler's Side Effects of Drugs (Sixteenth Edition), Elsevier Publisher, ISBN 9780444537164, pp.729-732, 2016.
Aparup Roy, "Thermal Conversion of Water`s Adhesive Force to Cohesive Force," International Journal of Scientific Research in Chemical Sciences, Vol.8, Issue.4, pp.19-22, 2021.
Yoshihisa Yamamoto, Kosuke Ohgi, Yoshinori Onuki, Toshiro Fukami, Tatsuo Koide, “Quality Evaluation of Humidified Magnesium Oxide Tablet Formulations with Respect to Disintegration Time Prolongation”, Chemical and Pharmaceutical Bulletin, Vol.71, Issue.2, pp.165-174, 2023.
Mori H, Tack J, Suzuki H. “Magnesium Oxide in Constipation”. Nutrients. Vol.13, Issue.2, pp.421, 2021.
United State Pharmacopoeia and National Formulary (USP-NF). Magnesium Oxide Tablets. Rockville, MD: United States Pharmacopeial Convention; Vol.43, Issue.6, 2024.
International Council for Harmonisation (ICH) of Technical Requirements for Pharmaceuticals for Human Use. Pharmaceutical Development Q8(R2). Geneva, Switzerland: International Council for Harmonisation (ICH) of Technical Requirements for Pharmaceuticals for Human Use; 2009.
International Council for Harmonisation (ICH) of Technical Requirements for Pharmaceuticals for Human Use Guideline. Validation of Analytical Procedures Q2(R2). Geneva, Switzerland: International Council for Harmonisation (ICH) of Technical Requirements for Pharmaceuticals for Human Use; 2023.
Reddy PS, Hotha KK, Sait S. “Complexity in Estimation of Esomeprazole and its Related Impurities' Stability in Various Stress Conditions in Low-Dose Aspirin and Esomeprazole Magnesium Capsules”. Scientia Pharmaceutica. Vol.81, Issue.2, pp.475-492, 2013.
Vikram Gharge, Anil Gadhe and Vikas Mohite et al. “Stability Indicating RP-HPLC Method for the Estimation of Impurities in Esomeprazole Gastro-Resistant Tablets by AQbD Approach”. BIOI. Vol.5, Issue.1, 2024.
Zdenek Spacil, Jana Folbrova, Nikolaos Megoulas, Petr Solich, Michael Koupparis, Simultaneous liquid chromatographic determination of metals and organic compounds in pharmaceutical and food-supplement formulations using evaporative light scattering detection, Analytica Chimica Acta, Vol.583, Issue.2, pp.239-245, 2007. ISSN 0003-2670.
Prachi Barsagade, Roshan Khetade, Kalyani Nirwan, Tikesh Agrawal, Santosh Gotafode, Upadesh Lade, Review Article of Dissolution Test Method Development and Validation of Dosage Form by using RP-HPLC, International Journal of Pharmaceutical Sciences Review and Research. Vol.70, Issue.1, pp.28-38, 2021.
S. Khalil, S. Alharthi, “Ion-selective Membrane Sensor for Magnesium Determination in Pharmaceutical Formulations”, International Journal of Electrochemical Science, Vol.15, Issue.9, pp.9223-9232, 2020. ISSN 1452-3981.
M. Aggrawal, J. Rohrer, “Magnesium oxide monograph modernization with ion chromatography”, Thermo Fisher Scientific Application note, Sunnyvale, CA. 2021.
S. Mandal, A. Alispahić, A. Dedić and H. Džudžević Čančar. “Spectrophotometric Determination of Magnesium Oxide Content in Supplements of Magnesium”, Kemija u industriji/Journal of Chemists and Chemical Engineers, Hurija. Vol.68, Issue.5-6, pp.197-200, 2019.
Kommareddy, S., Kumar, S., Nigam, R. S., & Mishra, D. K. Development and Validation of RP-HPLC Method for Dissolution Drug Release of Dimethyl Fumarate Delayed Release Capsules. Analytical Chemistry Letters, Vol.11, Issue.3, pp.409–426, 2021.
Inoue C, Kogure T, Kamata A, Ishihara M, Muraoka R, Tatsumi A, Tanaka K, Takeshita H, Hamaguchi T, Tagawa N, Kobayashi Y, Kadobayashi M. [Quality evaluation of magnesium oxide tablets using acid neutralization test and dissolution test]. Yakugaku Zasshi. Vol.127, Issue.12, pp.2085-9, 2007. PMID: 18057798
Anosh Charles, Neetu Trivedi, Anubha Vijay Pandya, "Repercussion of Routine Laboratory Practice in the Determination of Degree of Dissociation of Anion of Weak Electrolyte Using Corelation of Nernst Equation with Equivalent Conductance at Equilibrium," International Journal of Scientific Research in Chemical Sciences, Vol.9, Issue.6, pp.16-19, 2022.
United State Pharmacopoeia and National Formulary (USP-NF). Magnesium Oxide Tablets. Rockville, MD: United States Pharmacopeial Convention; USP 35 NF 30, pp.3752-3753, 2012.
Downloads
Published
How to Cite
Issue
Section
License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors contributing to this journal agree to publish their articles under the Creative Commons Attribution 4.0 International License, allowing third parties to share their work (copy, distribute, transmit) and to adapt it, under the condition that the authors are given credit and that in the event of reuse or distribution, the terms of this license are made clear.





